🇺🇸 USA
$SPX500 — Futures indicate a decisive surge, with the market attempting to recover losses from last week's banking sell-off.
$DJ30 — Futures in a solid rise, showing generalized risk-on sentiment.
$NSDQ100 — Futures are strongly up, with tech leading the market rebound.
💻 Tech & Growth Snapshot
$NVDA (-0,12%) — Up (0.55%), the stock is leading the semiconductor sector, confirming strong AI demand.
$GOOGL (+0,03%) — Up (0.19%), the stock joins the positive Nasdaq trend.
$AVGO (-0,02%) — Up (0.53%), the semiconductor sector benefits from renewed optimism.
$META (+0,32%) — Up (0.48%), showing a strong recovery after recent weakness.
$MSFT (+0,01%) — Up (0.36%), the stock regains momentum with positive sentiment.
$QBTS (+3,13%) — Strongly up, quantum computing sentiment has turned positive amid the tech rebound.
$RGTI (+0,34%) — Up sharply (3.08%), the quantum sector actively participates in the risk-on move.
$TSM (+2,27%) — Up sharply (2.43%), boosted by optimism in the semiconductor sector.
🛍️ Retail & Commerce
$AMZN (-0,5%) — Up (0.80%), strong pre-market recovery, led by tech.
$BABA (-0,42%) — Down (-0.57%), counter-trending Western tech, affected by Asian uncertainties.
$CVNA (+1,32%) — Up (0.57%), the stock gains ground following the broader market trend.
$SHOP (+1,09%) — Solidly up, retail tech is driven by the general risk-on mood.
⚕️ Health & Pharmaceutical
$LLY (+0,09%) — Up, tracking the general market rebound.
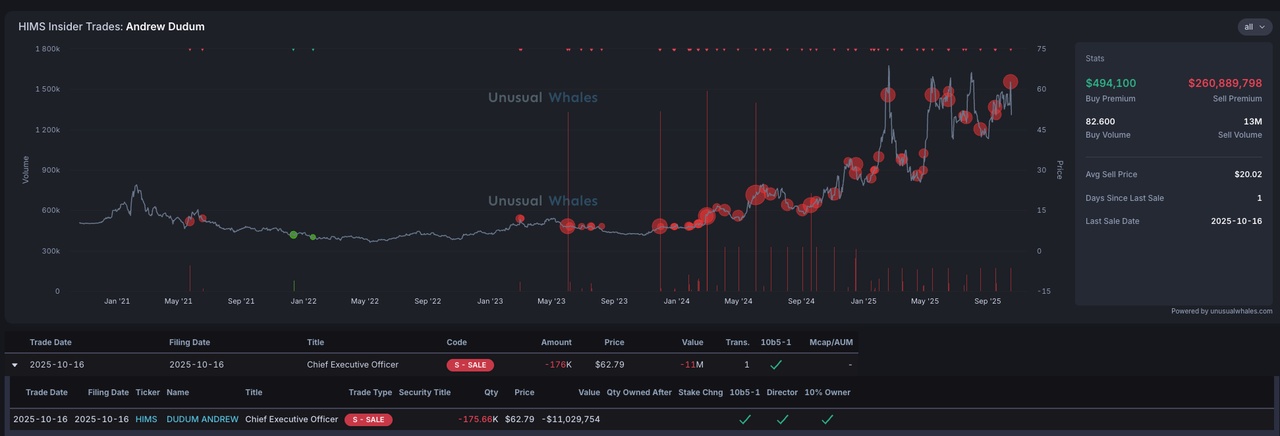
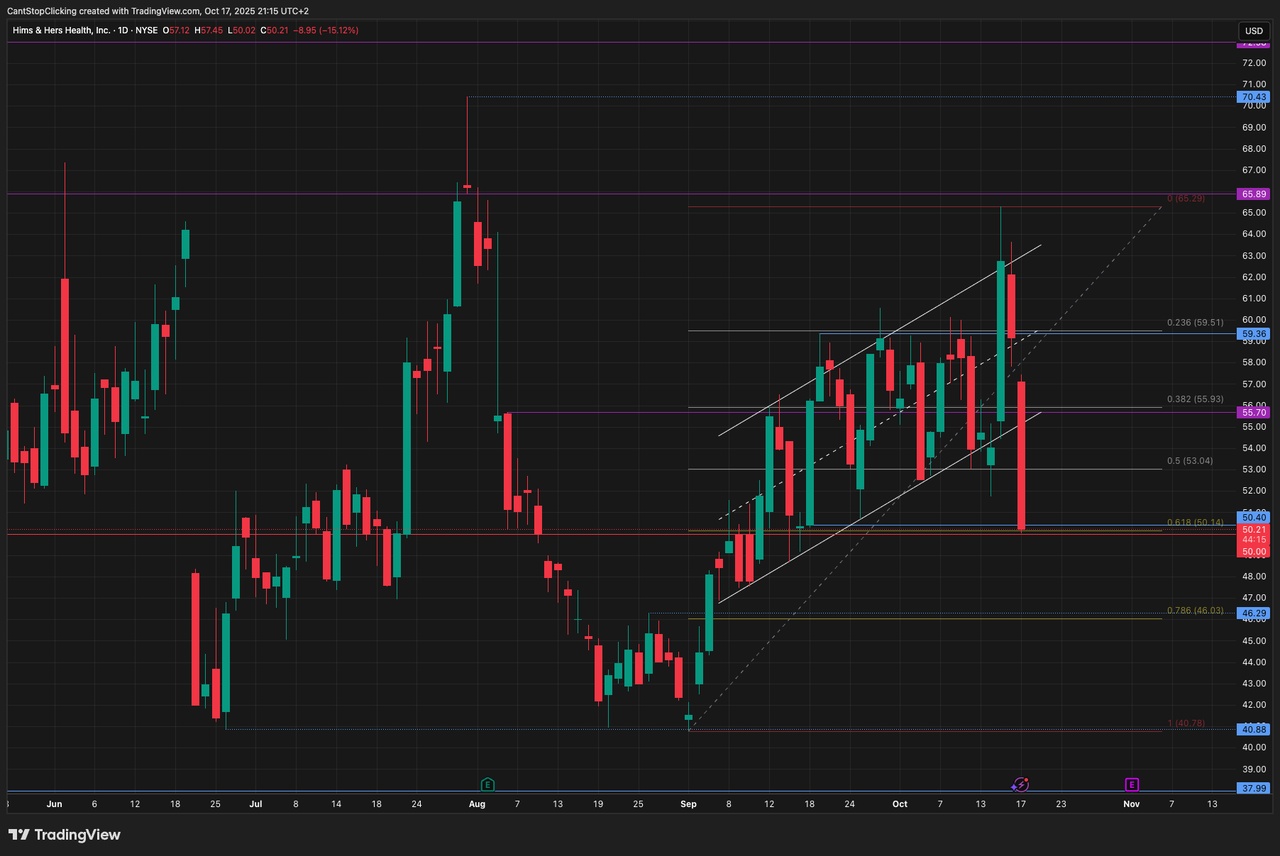
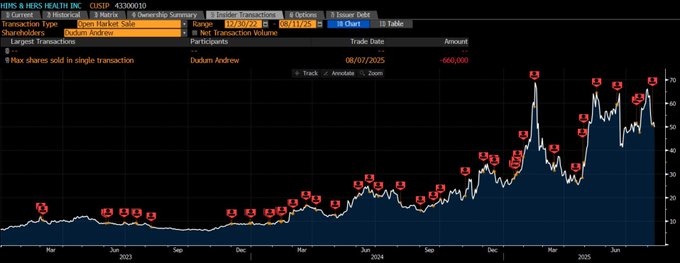
$HIMS (+3,02%) — Stable (0.00%), the stock is steady after last week's volatility.
$INSM (+1,06%) — Stable (0.00%), the biotech sector cautiously joins the rally.
🇪🇺 Europe
STOXX 600 — Opening solidly up, in line with global optimism.
GER40 — Decisively higher, the German market regains momentum.
$LDO (+2,77%) — Stable (0.00%), the defense sector is neutral in this rebound phase.
$$IBE (+0,85%) — Stable (0.00%), utilities are static in a risk-on environment.
$OKLO — Up sharply (1.73%), advanced nuclear technology continues its positive trend.
🏦 Banking & Finance
$$UCG (+0,99%) — Stable (0.00%), Italian banks are trying to establish a base after heavy selling.
$$ISP (+0,97%) — Stable (0.00%), awaiting clearer signals.
$$BAMI (+1,86%) , $CE (+0,38%) , $BPE (+2,76%) — Stable (0.00%), the financial sector shows caution despite the risk-on trend.
$$BBVA (+2,54%) — Stable (0.00%), the Spanish stock is leading the European banking recovery.
$AXP (+0,04%) — Up (0.59%), the payments sector participates in the rebound.
$V (+0,09%) — Up (0.07%), confirming its positive tone.
🌏 Asia
$JPN225 — Close in a solid rise, led by optimism in tech markets.
$KOSPI — Close up, Korean tech drives the index.
$HK50 — Up, tech stocks recover despite BABA's uncertainties.
$CHINA50 — Up, following positive global sentiment.
💱 Forex
$EURUSD — Up, the Dollar is losing momentum in a risk-on phase.
$GBPUSD — Up, the market positively assesses prospects for a stronger economy.
$USDJPY — Down, the Yen is gaining ground.
$DXY — The Dollar Index is showing clear weakness.
💎 Commodities & Precious Metals
$GLD (+0,42%) — Down slightly (0.00%), gold consolidates as investors shift to riskier assets.
$CDE (+0,88%) — Stable (0.00%), tracking the flat movement of gold.
$BRENT — Up, showing signs of demand recovery.
$WTI — Gaining ground, reflecting positive macroeconomic sentiment.
📈 Benchmark ETFs
$VOO (+0,27%) — Tracking $SPX500$ futures higher.
$VGT (+0,54%) — Up (0.00%), reflecting the strength of the technology sector.
$$CSNDX (+0,3%) — Up (0.00%), tracking Nasdaq futures in positive territory.
$BND (-0,04%) — Down (0.00%), reflecting rising yields.
💰 Crypto
$BTC (+1,8%) — Strong recovery, the crypto sector bounces off the bottom and gains ground.
$ETH (+1,04%) — Solidly up, following Bitcoin.
$TRX (+1,04%) — Up (0.00%), the altcoin sector participates in the rally.
$CRO (+2,7%) — Up, in line with overall positive sentiment.
🚀 Space & New Tech
$RKLB (+3,95%) — Up, sentiment for growth stocks suggests a rebound.
🔎 Deep Dive: The Return of Risk-On
The week opens with a decisive "Risk-On" mood. Markets are clearly shrugging off (for now) last week's banking tensions, focusing instead on tech-led growth ($NVDA, $TSM$) and hopes for monetary easing. The strong rally in cryptocurrencies ($BTC, $ETH$) and the weakness of the Dollar ($DXY$) are clear indicators that liquidity is flowing back into riskier assets. European banks ($BBVA.MC$) and the semiconductor sector show unexpected strength, while gold ($GLD$) pauses, confirming the shift in focus from systemic risk to growth opportunities.
For daily real-time market insights, deep dives, and trading discussions, follow me on X: https://x.com/ThomasVioli
To copy my portfolio, strategies, and complete trade insights, you can follow me on eToro: https://www.etoro.com/people/farlys
⚠️ Disclaimer: Past performance is not indicative of future results. Investing involves risks, including the loss of capital.