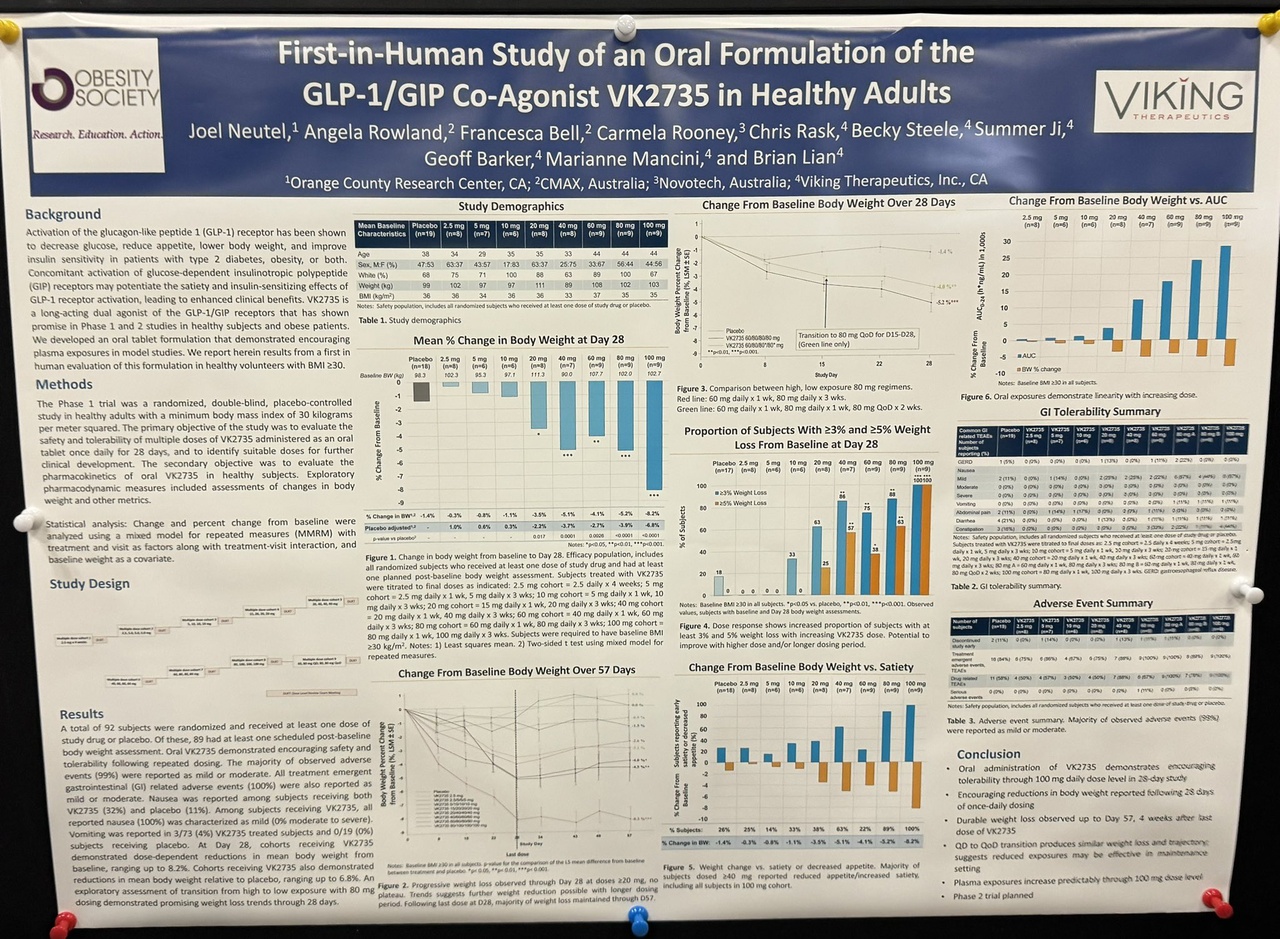
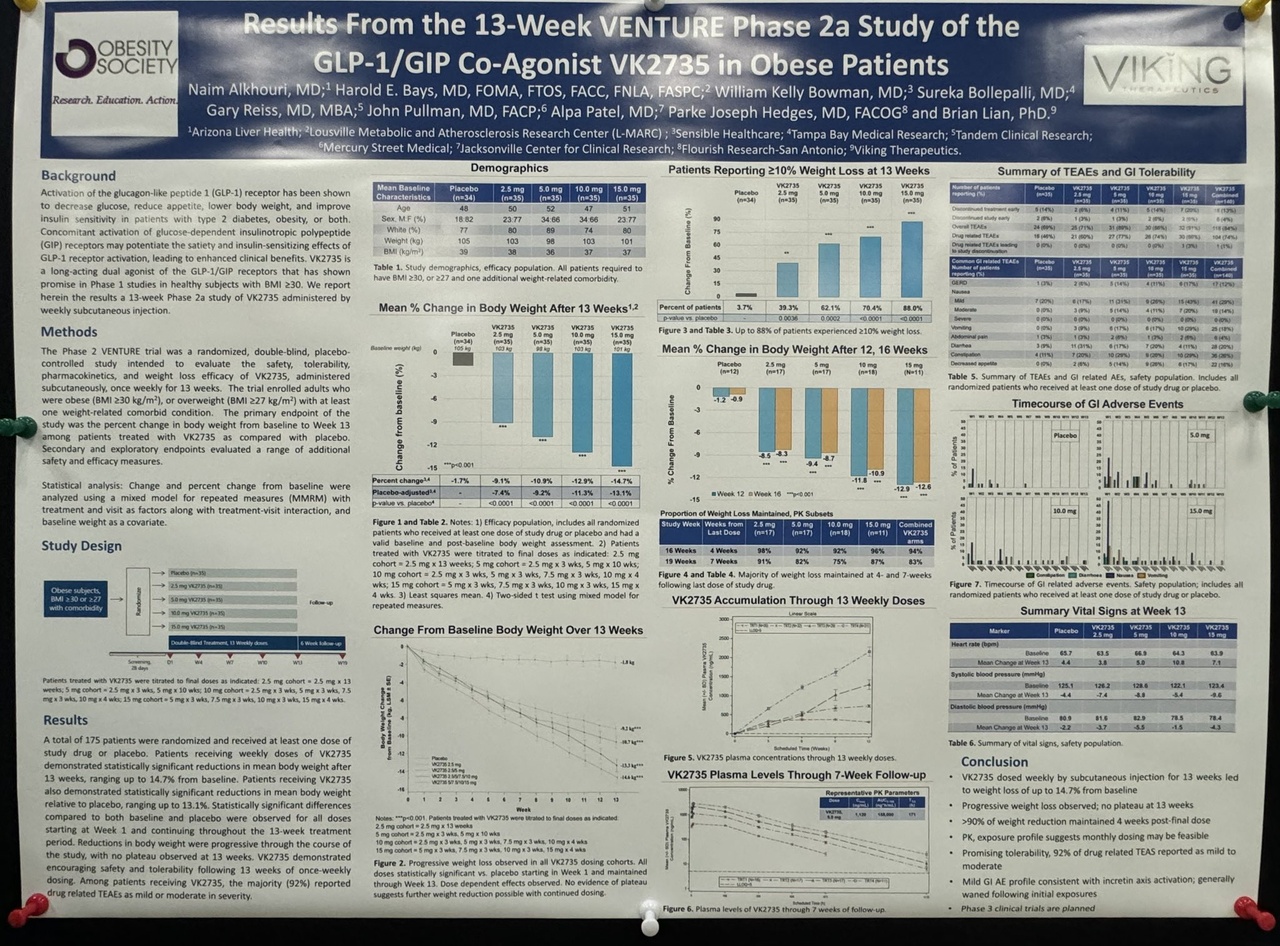
I assume that if $LLY (+1,54 %) and $NOVO B (+0,01 %) do not follow suit $VKTX (+3,58 %) will become the new market leader.
It could even be that Viking will be the only provider of an effective and well-tolerated weight loss pill for a long time.
Viking is very likely to bring the next two really big blockbuster drugs onto the market. Since the market has been waiting for such blockbusters for a long time, the valuation will be correspondingly high.
Should the share price fall again, take a look at all the GLP-1 pipelines and Viking.