In the last few days there have been news about Sangamo Therapeutics ($SGMO (+0,3 %)), which for me are more than just headlines:
➡️ a new patent for the further development of zinc finger technology
➡️ and a sign of life from the paused TX200 program
Both show that Sangamo continues to expand its platform - quietly but steadily. ☝️
1️⃣ New patent: "Engineered Genetic Modulators"
On October 9, 2025 a new patent was published at the US Patent and Trademark Office (USPTO):
US20250313848 - "Engineered Genetic Modulators" which you can view here 👉https://tinyurl.com/3e8d2bpr
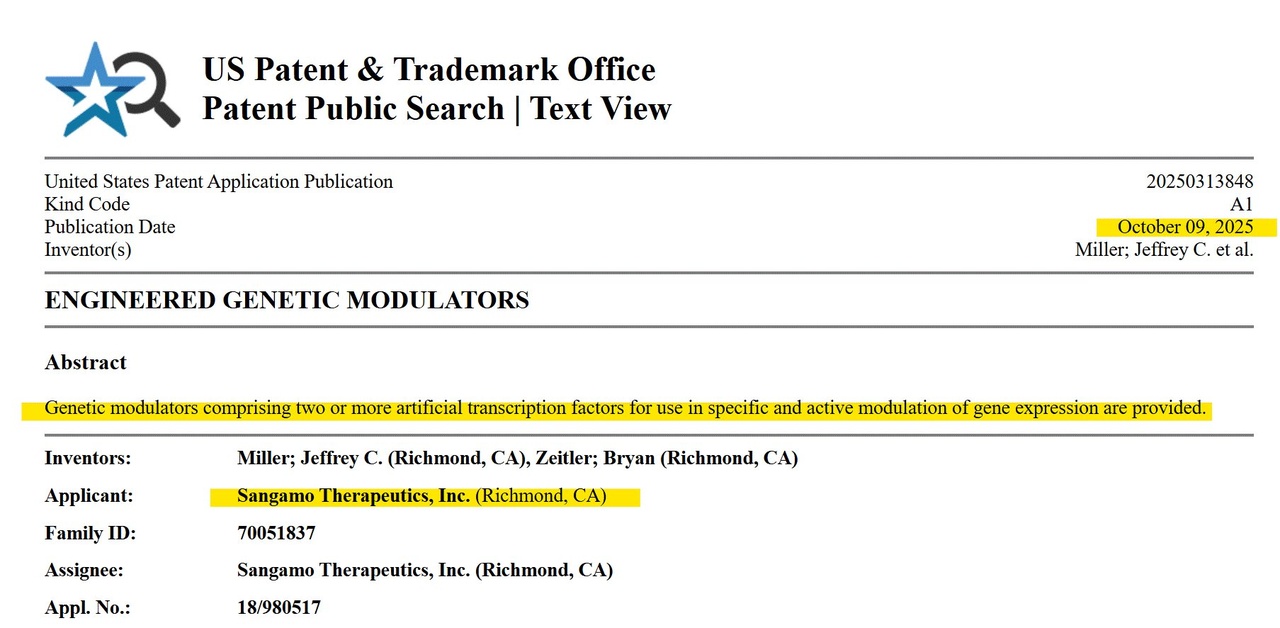
(the original patent US20200109406 from April 9, 2020 can be found here 👉 https://tinyurl.com/mry9rhxp - If the two links do not work, simply go to https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html and enter the respective patent number without the suffix "US" in the search field)
This new patent extends Sangamo's Zinc Finger Platform (ZFP) - the molecular tools the company uses to controls genesinstead of cutting them cutting them up.
While many competitors still rely on CRISPR a technology that permanently alters DNA - Sangamo has long been working with a more precise, controllable systemthat modifies genes like a dimmer instead of irrevocably rewriting them. This is an enormous safety advantage, especially in the nervous system.
In short: CRISPR cuts - Sangamo directs. And this is exactly what the next generation of patents is being created for - a new era of controllable gene therapy.
2️⃣ TX200 - the "paused" program lives on
Almost at the same time there was a sign of life on LinkedIn:
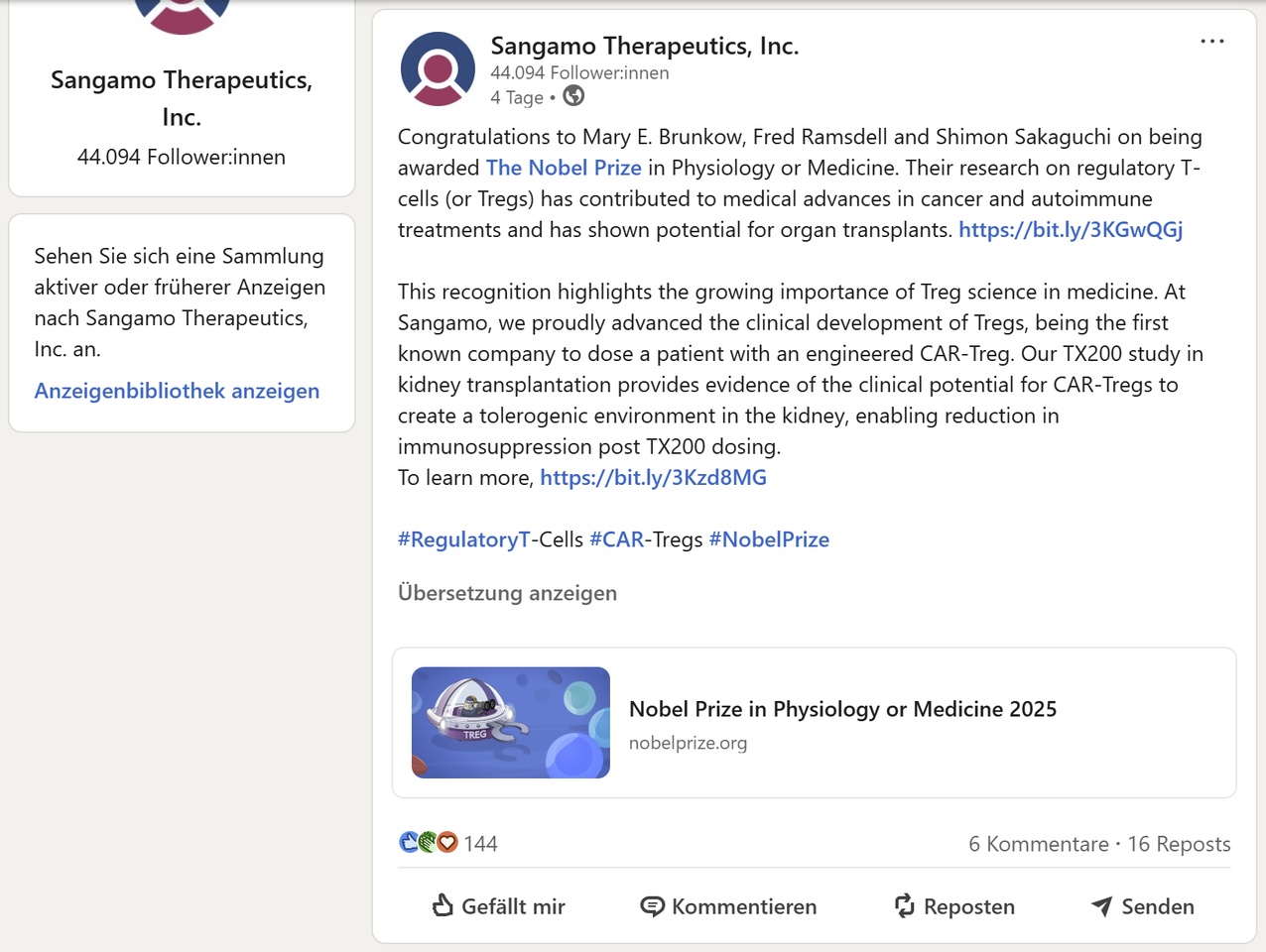
Sangamo shows there that the CAR-Treg program TX200 - developed to prevent rejection reactions after kidney transplants - is still scientifically active.
💡 The special feature: TX200 was the world's first clinically approved CAR-Treg program. Sangamo was therefore a pioneer - and made clinical history long before other players emerged in this field.
Pausing this program was not a setback, but a strategic decision: The program is expensive, complex and needs partner capital - but scientifically it is fully intact. Instead of suppressing the immune system, it is "retrained" to accept the donor organ - a completely new immunological approach.
The Nobel Prize and the fact that Sangamo is now taking up the topic again publicly shows that the platform is alive - and remains relevant: The platform is alive - and remains relevant.
🧠 Background: The zinc finger platform - Sangamo's silent backbone
If you want to understand Sangamo, you have to understand the technology. The company works with zinc finger proteins (ZFPs) - tiny DNA binders that precisely switch genes switch genes on or off, correct or regulate them. or regulate genes. Ingenious!
This results in three pillars of application:
1️⃣ Gene regulation - Switching genes on or off in fine doses (e.g. ST-503, ST-506, tauopathies with Genentech)
2️⃣ Gene editing - Correct DNA sequences permanently (e.g. sickle cell)
3️⃣ CAR-Tregs - Programming immune cells (e.g. TX200)
And in order for these therapies to reach the brain at allSangamo is also developing its STAC-BBB delivery platform - a kind of molecular transporter that carries genetic therapies across the blood-brain barrier.
This platform is unique in the industry and is already being used by:
- Genentech / Roche (tauopathies)
- Astellas (exclusive license for up to five CNS targets)
- Eli Lilly (parallel license package with up to USD 1.4 billion milestone potential)
Sangamo earns from license fees, milestones and royaltieseven when others are in the spotlight.
🎯 Conclusion - Two small pieces of news, but one big pattern:
Sangamo remains true to its line - focused, data-driven and long-term. Not a hype story, but an example, how quiet pioneers are building the future step by step.
💭 And one final thought
Now there are visible triggers - and that changes everything:
🔹 The new patent confirms the further development of the platform
🔹 TX200 shows activity again
🔹 ST-503 starts clinically
🔹 Fabry is about to be approved
🔹 Further partnerships are on the horizon
Sangamo has never had this combination before. It looks to me like the company is on the verge of a real revaluation - scientifically, strategically and perhaps now also on the stock market again 🤑😜
What fascinates me again and again: Sangamo has the technology that others are still looking for. While companies like CRISPR Therapeutics ($CRSP) (-1,1 %) or Intellia ($NTLA) (-0,51 %) are being criticized for their gene scissors ✂️gefeiert, Sangamo has long been working on the next generation - more more precise, safer, reversible. Not a one-way street in the genome, but real control.
Looking back, I think it's fair to say that Sangamo was ahead of its time - a pioneer when the world wasn't even ready. Today, everything fits together: Data, platform, partners, momentum. When the market finally recognizes this, we won't be talking about percentages - but about breakthroughs that make history. 🚀